Q.
For the ‘greenhouse effect’ consider statements I to III.
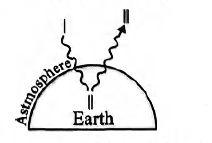
(I) Sunlight is received by Earth. Some incoming radiations are reflected back into space by the atmosphere and some is absorbed such as certain $UV$ light by stratosphere ozone
(II) Earth’s surface emits $IR$ radiations.
(III) Emitted $IR$ radiations are less intense than that emitted by Earth’s surface. $CO_2$ along with $IR$ radiations warm the atmosphere.
Select the correct statements.
Environmental Chemistry
Solution: