Q.
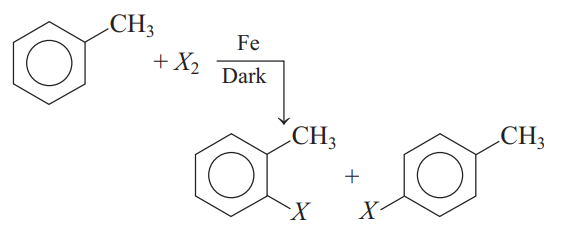
For the above reaction
I. $X= Cl$; ortho and para-isomers can be separated out
II. $X= I$; reaction does not occur due to high reactivity of iodine
III. $X= F$; reaction with fluorine is reversible.
Select the most appropriate option with respect to correct statements.
Haloalkanes and Haloarenes
Solution: