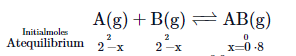Q. For reaction $A(g) + B(g)\rightleftharpoons AB(g)$ we start with $2$ moles of $A$ and $B$ each. At equilibrium $0.8$ moles of $AB$ is formed. Then how much of $A$ changes to $AB$
Equilibrium
Solution:
Solution: