Q.
A reactant (A) forms two products :
$E_{ a _{2}}=2 E_{ a _{1}}$
Frequency factors for both the reactions are equal. Therefore,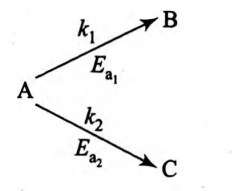
Chemical Kinetics
Solution: