Q.
For a reaction,
$N_2(g) + 3H_2(g) \rightarrow 2NH_3(g)$; identify dihydrogen $(H_2)$ as a limiting reagent in the following reaction mixtures.
Solution:
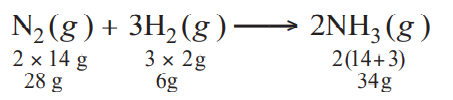 ...(I)
...(I)