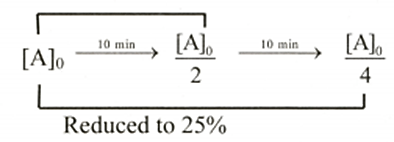
Q. For a first order reaction, the half-life is $\text{10} \, \text{mins} \text{.}$ How much time in minutes will it take to reduce the concentration of reactant to $25\%$ of its original concentration?
NTA AbhyasNTA Abhyas 2022
Solution: