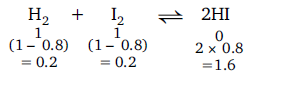Q. Equivalent amounts of $H_2$ and $I_2$ are heated in a closed vessel till equilibrium is obtained. If 80% of the hydrogen can be converted to $HI$, the $K_c$ at this temperature is
VITEEEVITEEE 2007
Solution: