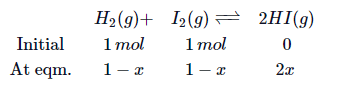Q. Equimolar concentrations of $H_2$ and $I_2$ are heated to equilibrium in a $2$ litres flask. At equilibrium, the forward and the backward rate constants are found to be equal. What percentage of initial concentration of $H_2$ has reacted at equilibrium ?
Equilibrium
Solution: