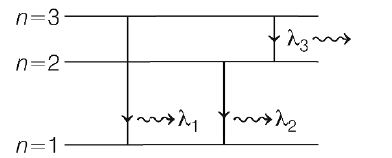
Q. Due to transitions among its first three energy levels, hydrogenic atom emits radiation at three discrete wavelengths $\lambda_{1}\lambda _{2}$ and $\lambda_{3}\left(\lambda_{1} <\lambda_{2}< \lambda_{3} \right)$. Then
KVPYKVPY 2011Atoms
Solution: