Q.
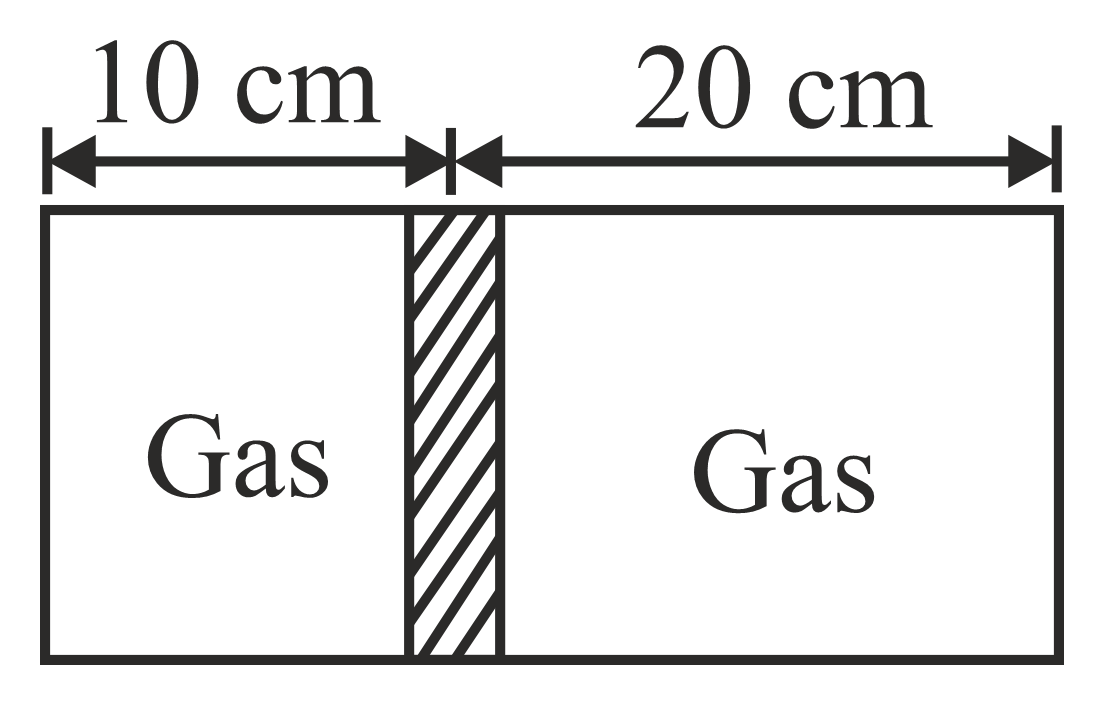
Diagram shows a horizontal cylindrical container of length $30cm$ , which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in the diagram. The temperature of left part of cylinder is $100K$ and that on right part is $400K$ . Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator after a long when gases on the two parts of cylinder are in thermal equilibrium.
NTA AbhyasNTA Abhyas 2022
Solution: