Q.
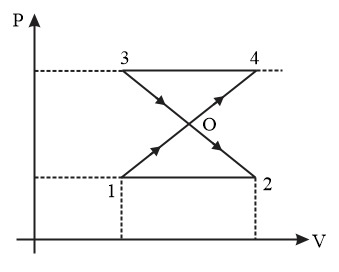
Determine the work done by an ideal gas undergoing a cyclic process from $1 \rightarrow 4 \rightarrow 3 \rightarrow 2 \rightarrow 1$ . Given $P_{1}=10^{5} \, Pa, \, P_{0} \, = \, 3\times 10^{5} \, Pa, \, P_{3}=4\times 10^{5} \, Pa$ and $V_{2}-V_{1}=10 \, L$ .
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: