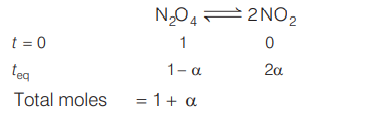Q.
Density of equilibrium mixture of $N _{2} O _{4}$ and $NO _{2}$ at $1 \,atm$ and $384 \,K$ is $1.84 \,g / dm ^{3}$. Equilibrium constant of the following reaction is
$N _{2} O _{4} \rightleftharpoons 2 NO _{2}$
AIIMSAIIMS 2016
Solution: