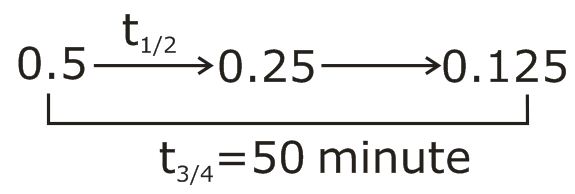Q. Decomposition of $H_{2}O_{2}$ follows a first order reaction. In fifty minutes the concentration of $\text{H}_{2} \text{O}_{2}$ decreases from $0.5$ to $0.125 \, M$ in one such decomposition. When the concentration of $H_{2}O_{2}$ reaches $0.05 \, M$ , the rate of formation of $O_{2}$ will be:
NTA AbhyasNTA Abhyas 2020Chemical Kinetics
Solution: