Q.
$Cu ^{+2}+2 e ^{-} \rightarrow Cu ; \log \left[ Cu ^{+2}\right]$ vs $E _{ RP }$ graph is of the type as shown in figure where $OA =0.34$ volt. Then electrode potential of the half cell of $Cu / Cu ^{+2}(0.1\, M )$ will be:-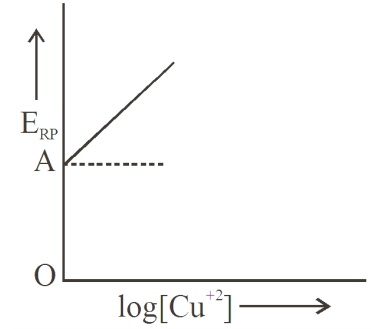
Solution: