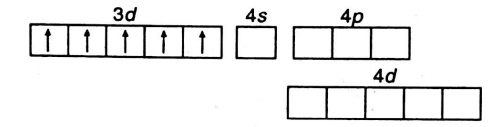
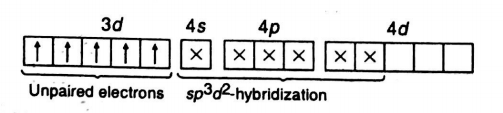
Q. Considering $ {{H}_{2}}O $ as weak field ligand, the number of unpaired electrons in $ {{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}} $ will be (At. no. of $ Mn=25 $ )
ManipalManipal 2007Coordination Compounds
Solution:
Solution: