Q.
Consider the reaction :
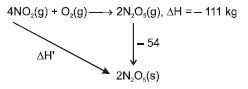
$4 NO _{2}( g )+ O _{2}( g ) \rightarrow 2 N _{2} O _{5}( g )$
$\Delta_{r} H =-111 kJ$
If $N _{2} O _{5}( s )$ is formed instead of $N _{2} O _{5}( g )$ in the above reaction, the $\Delta_{r} H$ value will be :
(given, $\Delta H$ of sublimation for $N _{2} O _{5}$ is $54 kJ mol ^{-1}$ )
AIEEEAIEEE 2011Thermodynamics
Solution:
$- 111 - 54 = \Delta H'$
$\Delta H' =- 165 \,KJ$