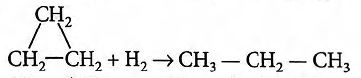Q.
Consider the following two reactions :
(i) Propene $+ H_2 \to$ Propane ; $\Delta H_1$
(ii) Cyclopropane $+ H_2$ Propane ; $\Delta H_2$
Then, $\Delta H_2 - \Delta H_1$ will be
Thermodynamics
Solution: