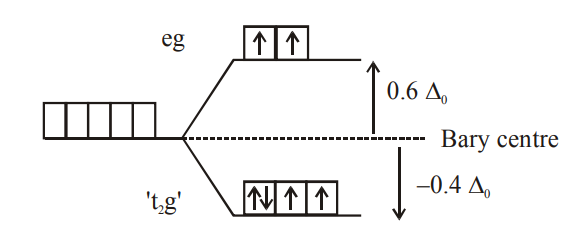
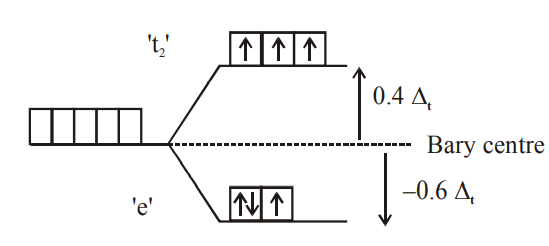
Q. Consider that a $d ^{6}$ metal ion $\left( M ^{2+}\right)$ forms a complex with aqua ligands, and the spin only magnetic moment of the complex is $4.90 BM$ The geometry and the crystal field stabilization energy of the complex is :
Solution:
Solution: