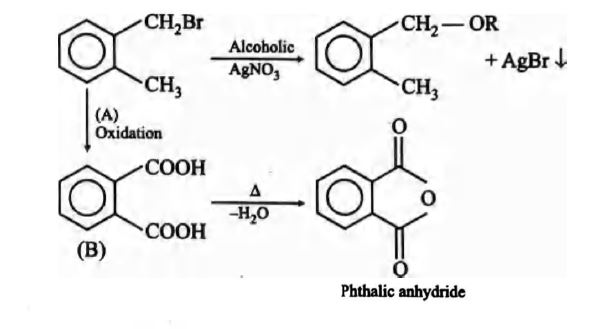
Q. Compound (A), $C_{8}H_{9}Br$ gives a yellow precipitate when warmed with alcoholic $AgNO_{3}$. Oxidation o f (A) gives an acid (B), $C_{8}H_{6}O_{4}$ . (B) easily forms anhydride on heating. Identify the compound (A).
Haloalkanes and Haloarenes
Solution: