Q.
Column I
Column II
A
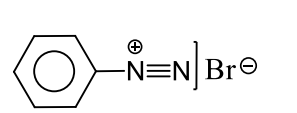
p
Sodium fusion extract of the compound gives Prussian blue colour with $FeSO _4$
B
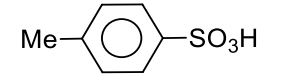
q
Sodium fusion extract of the compound gives blood red colour with $FeSO _4$
C
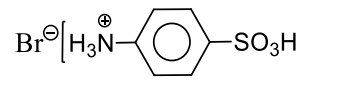
r
Lassaigne's extract (L.E.) in $CS _2$ and $Cl _2$ water gives orange colour
D
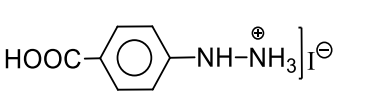
s
L.E. with $\left[ Fe ( CN )_5 NO \right]^{2-}$ gives violet colour
| Column I | Column II | ||
|---|---|---|---|
| A |  |
p | Sodium fusion extract of the compound gives Prussian blue colour with $FeSO _4$ |
| B |  |
q | Sodium fusion extract of the compound gives blood red colour with $FeSO _4$ |
| C |  |
r | Lassaigne's extract (L.E.) in $CS _2$ and $Cl _2$ water gives orange colour |
| D |  |
s | L.E. with $\left[ Fe ( CN )_5 NO \right]^{2-}$ gives violet colour |
Organic Chemistry – Some Basic Principles and Techniques
Solution: