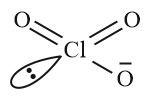
Q. Chlorine reacts with hot and concentrated NaOH and produces compounds (X) and (Y). Compound (X) gives white precipitate with silver nitrate solution. The average bond order between $Cl$ and $O$ atoms in (Y) is ______ .
NTA AbhyasNTA Abhyas 2022
Solution: