Q.
Calculate the standard cell potential in(V) of the cell in which following reaction takes place :
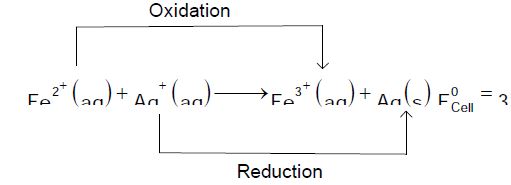
$Fe^{2+}\left(aq\right)+Ag^{+}\left(aq\right)\rightarrow Fe^{3+}\left(aq\right)+Ag\left(s\right)$
Given that
$E^{\circ}_{Ag^{+}}/_{Ag} =xV$
$E^{\circ}_{Fe^{2+}}/_{Fe} =yV$
$E^{\circ}_{Fe^{3+}}/_{Fe} =zV$
Solution:
Given:
$E^{0}_{ Ag'/Ag} = x \quad---- \left(1\right)$
$E^{0}_{ Fe^{2+} /Fe} = y\quad----\left(2\right)$
$E^{0} _{Fe^{2+} /Fe} = z \quad----\left(3\right)$
Using equation:
$\Delta G^{0} = -nFE^{0}$
$\Delta G^{0}_{1} = -fx$
$\Delta G^{0}_{2} = -2Fy$
$\Delta G^{0}_{3} = - 3Fz$
$Fe^{2+} 2e^{-} \to Fe - 2Fy$
$Fe_{_-}^{3+} + 3e_{_-}^{-} \to Fe_{_-} - 3F_{_+}z$
$Fe^{2+} \to Fe^{3+} + e^{-} \left(-2Fy + 3Fz\right)$
$Ag^{+} + e^{-} \to Ag - Fx$
$\Delta G_{Total} = -2Fy+3Fz-Fx = -FE^{0}_{Cell}$
$E^{0}_{Cell} = x+2y-3z$