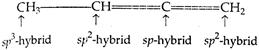Q. $ C{{H}_{3}}\cdot CH=C=C{{H}_{2}} $ has:
JIPMERJIPMER 1997
Solution:
Carbon atom, whose valencies are satisfied with 4 single bonds, is $ s{{p}^{3}}\text{-} $ hybrid. Carbon atom, whose valencies are satisfied with one double-bond, is $ s{{p}^{2}}\text{-} $ hybrid while the carbon atom, whose valencies are satisfied either by 2 double-bonds or one triple and one single bond, is $ sp\text{-} $ hybrid.