Q.
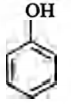 $+C_{2}H_{5}I \xrightarrow[{{\text{Anhydrous}} (C_{2}H_{5}OH)}] {{^{-}OC_{2}H_{5}}}$?
$+C_{2}H_{5}I \xrightarrow[{{\text{Anhydrous}} (C_{2}H_{5}OH)}] {{^{-}OC_{2}H_{5}}}$?
Alcohols Phenols and Ethers
Solution: