Q.
Before equilibrium is set-up for the chemical reaction, $N_2O_4 \rightleftharpoons 2NO_2$, vapour density of the gaseous mixture was measured. If $D$ is the theoretical value of vapour density, variation of $x$ with $D/d$ is shown by the graph.
What is value of $D/d$ at point $Y$?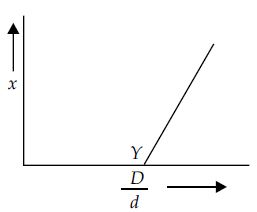
Equilibrium
Solution: