Q. Basicity of orthophosphoric acid is
AIPMTAIPMT 1991The p-Block Elements - Part2
Solution:
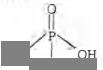
Orthophosphoric acid $(H_3PO_4)$ have the
following structure
It is clear from the structure that it contains three
replaceable hydrogen atoms, so it gives three H+
ions on dissolution in water. So, the basicity of
$H_3PO_4$ is three
$\, \, \, \, \, \, \, H_3PO_4 \, \longrightarrow \, 3H^+ + PO^{3-}_4 $