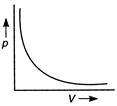
Q. At constant temperature, the curve between p and V is
Rajasthan PETRajasthan PET 2007
Solution:
According to Boyle's law, at constant temperature the volume of a given mass of a gas is inversely proportional to its pressure $ p\propto \frac{1}{V} $ $ pV= $ constant At constant temperature, on plotting a graph between p and V, a hyperbola curve is obtained.