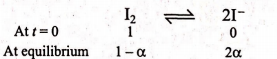Q. At $730^{\circ} C$ the vapour density of iodine under a pressure of $1$ atm is $97.45 \%$ of the theoretical value, on the assumption that the molecule of gaseous iodine is diatomic. What is the degree of dissociation of $I _{2}$ ?
Equilibrium
Solution: