Q.
Assertion : The bond angle 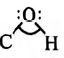 in alcohols is slightly less than the tetrahedral angle.
in alcohols is slightly less than the tetrahedral angle.
Reason : In alcohols, the oxygen of the $—OH$ group is attached to carbon by a sigma bond formed by the overlap of a $sp^3$ hybridised orbital of carbon with $sp^3$ hybridised orbital of oxygen.
Alcohols Phenols and Ethers
Solution:
 in alcohols is slightly less than the tetrahedral angle due to the repulsion between the unshared electron pairs of oxygen.
in alcohols is slightly less than the tetrahedral angle due to the repulsion between the unshared electron pairs of oxygen.