Q.
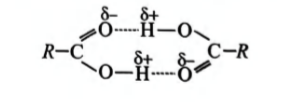
Assertion: Most carboxylic acids exist as dimers in the vapour phase or in aprotic solvents.
Reason : Higher carboxylic acids are practically insoluble in water due to the increased hydrophobic interaction of hydrocarbon part.
Aldehydes Ketones and Carboxylic Acids
Solution: