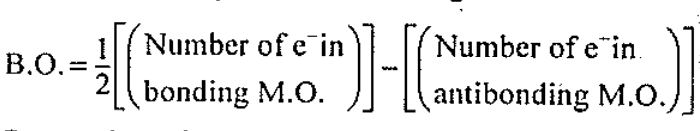Q.
Assertion: Bond order in a molecule, which can assume any value positive or negative, integral or fractional including zero.
Reason: Bond order depends on the number of electrons in the bonding and antibonding orbitals
AIIMSAIIMS 1998
Solution: