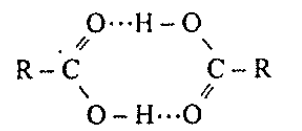Q.
Assertion : Boiling and melting points of amides are higher than corresponding acids.
Reason : It is due to strong intermolecular hydrogen bonding in their molecules.
Solution: