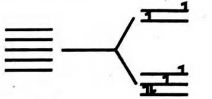
Q.
Arrange the following in order of decreasing number of unpaired electrons?
I. $\left[ Fe \left( H _{2} O \right)_{6}\right]^{2+}$
II. $\left[ Fe ( CN )_{6}\right]^{3-}$
III. $\left[ Fe ( CN )_{6}\right]^{4-}$
IV. $\left[ Fe \left( H _{2} O \right)_{6}\right]^{3+}$
Coordination Compounds
Solution: