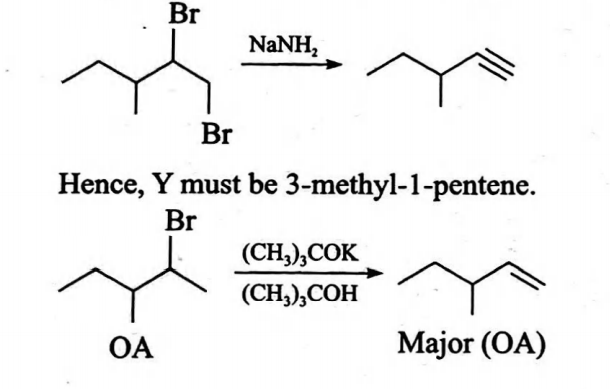
Q. An organic compound $X \left( C _{6} H _{13} Br \right)$ is optically active. $X$ on treatment with $\left( CH _{3}\right)_{3} COK$ in $\left( CH _{3}\right)_{3} COH$ gives $Y$ $\left( C _{6} H _{12}\right)$, a major product. $Y$ on treatment with $Br _{2}- CCl _{4}$ in the presence of $FeBr _{3}$ gives a dibromide which on further treatment with $NaNH _{2}$ gives $C _{6} H _{10}$ which is still optically active. Hence, $X$ and $Y$ respectively are
Hydrocarbons
Solution: