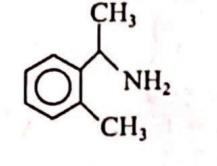
Q. An organic compound $(A) C _{9} H _{13} N$ dissolves in dil. $HCl$ and releases $N _{2}$. with $HNO _{2}$ giving an optically active alcohol. Alcohol on oxidation gives dicarboxylic acid, which on heating form anhydride. The organic compound $(A)$ is
Amines
Solution: