Q. An octahedral complex is formed when hybrid orbitals of the following type are involved
Solution:
| Hybridisation | Geometry of complex |
|---|---|
| $sp^3$ | tetrahedral |
| $ dsp^2$ | square planar |
| $d^2sp^3 $ | octahedral |
| $sp^2d^2 $ | not possible |
Solution:
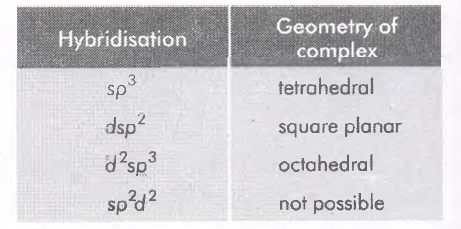
| Hybridisation | Geometry of complex |
|---|---|
| $sp^3$ | tetrahedral |
| $ dsp^2$ | square planar |
| $d^2sp^3 $ | octahedral |
| $sp^2d^2 $ | not possible |
