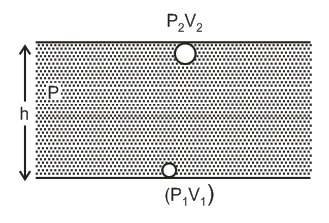
Q. An inverted bell, lying at the bottom of lake $47.6 \, m$ deep, has $50 \, cm^{3}$ of air trapped in it. The bell is brought to the surface of the lake, then the volume of the trapped air will become [atmospheric pressure = $70 \, cm$ of $Hg$ and density of $Hg=13.6 \, g \, cm^{- 3}$ ]
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: