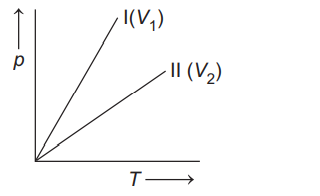
Q. An ideal gas of given mass is heated first in a small vessel (I) and then in a large vessel (II). A plot between $p\, v s \,T$ are plotted for (I) and (II). The correct $p-T$ curves in I and II conditions is
States of Matter
Solution:
In vessel (I), volume is small $\left(V_{1}\right)$,
while in second vessel volume is large $\left(V_{2}\right)$.
From Gay Lussac's law, $p \propto T($ at constant $V)$
The correct $p$ - $T$ plot is given below.