Q.
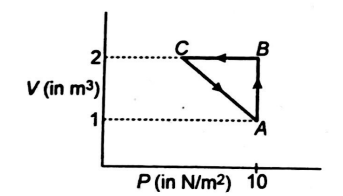
An ideal gas is taken through the cycle $ A\to B\to C\to A, $ as shown in figure. If the net heat supplied to the gas in the cycle is $5 \,J$, the work done by the gas in the process. $ C\to A $ is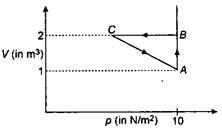
Rajasthan PMTRajasthan PMT 2007Thermodynamics
Solution: