Q.
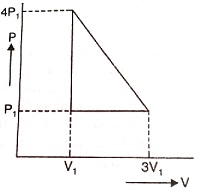
An ideal gas is taken through a series of changes, from $P_{1},V_{1} \rightarrow 4P_{1},V_{1} \rightarrow P_{1},3V_{1}$ represented in the figure. The net work done by the gas at the end of the cycle is equal to
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: