Q. An ideal gas expands isothermally from a volume $V_1$ to $ V_2$and then compressed to original volume $V_1$ adiabatically. Initial pressure is p$_1$ and final pressure is p$_3$. The total work done is W. Then,
IIT JEEIIT JEE 2004Thermodynamics
Solution:
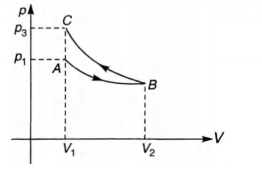
Slope of adiabatic process at a given state (p, F, T) is more than the slope of isothermal process. The corresponding p-V graph for the two processes is as shown in figure.
In the graph, AB is isothermal and BC is adiabatic.
W$_{AB}$ = positive (as volume is increasing)
and $W_{BC}$ = negative (as volume is decreasing) plus,
$|W_{BC}| > |W_{AB}|$ as area underp- Vgraph gives the work done.
Hence, W$_{AB}$ + W$_{BC}$ = W < 0
From the graph itself, it is clear that p$_3$ > p$_v$.
Hence, the correct option is (c).
NOTE At point B, slope of adiabatic (process BC) is greater than the slope of isothermal (process AB).