Q.
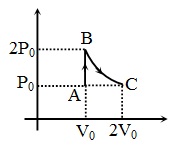
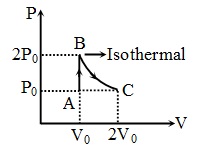
An ideal diatomic gas undergoes a thermodynamic process as shown in the $P-V$ diagram. The process $AB$ is isochoric while the process $BC$ is isothermal. The total heat given to the gas in the process is nearly (use $ln2 \, = \, 0.7$ )
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: