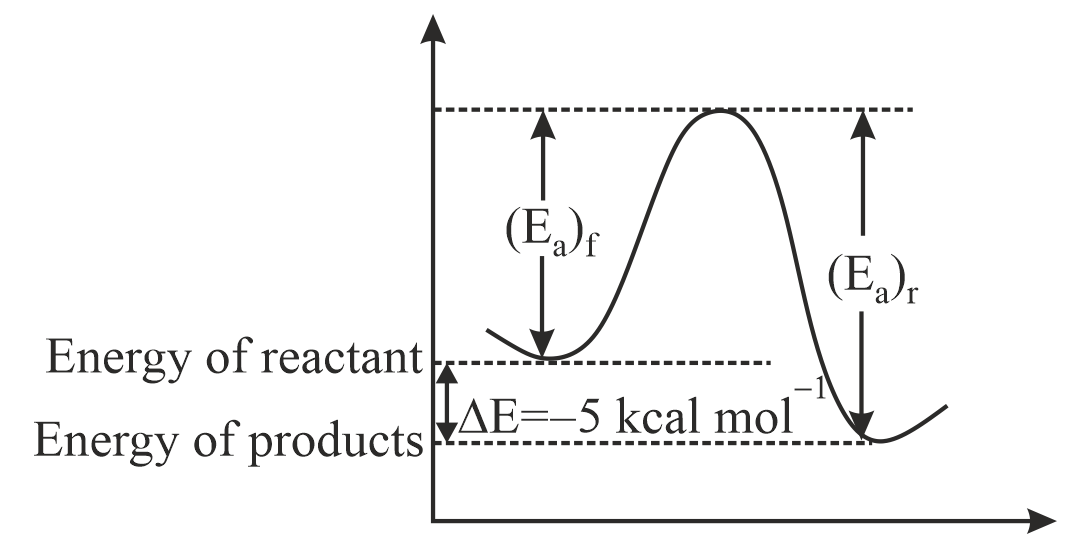
Q. An exothermic reaction, $A \rightarrow B$ , has an activation energy of $15kcalmol^{- 1}$ and the energy of reaction is $5kcalmol^{- 1}$ . The activation energy in $kcalmol^{- 1}$ for the reaction, $B \rightarrow A$ is
NTA AbhyasNTA Abhyas 2022
Solution: