Q.
An exothermic chemical reaction proceeds by two stages.
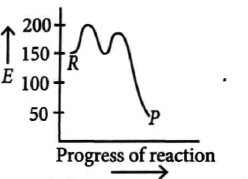
Reactants $\xrightarrow{\text{Stage}\, 1}$ Intermediate $\xrightarrow{ \text{Stage}\, 2 }$ Products The activation energy of state $I$ is $50 \,kJ$ $mol^{-1}$. The overall enthalpy change for the reaction is $-100 \,kJ$ $mol^{-1}$. Which diagram could represent the energy level diagram for the reaction?
AMUAMU 2014Chemical Kinetics
Solution: