Q.
An engine operates by taking $n$ moles of an ideal gas through the cycle $ABCDA$ shown in figure. The thermal efficiency of the engine is :
(Take $C_v = 1.5 \, R,$ where $R$ is gas constant)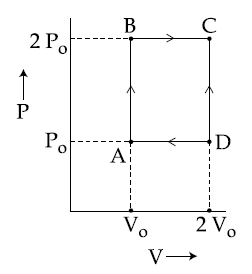
Solution: