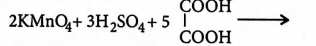Q. Amount of oxalic acid present in a solution can be determined by its titration with $KMnO_{4}$ solution in the presence of $H_{2}SO_{4}$. The titration gives unsatisfactory result when carried out in the presence of $HCl$, because $HCl$
Redox Reactions
Solution: