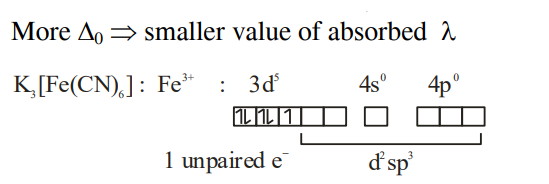
Q. Amongst $FeCl _{3} .3 H _{2} O , K _{3}\left[ Fe ( CN )_{6}\right]$ and $\left[ Co \left( NH _{3}\right)_{6}\right] Cl _{3}$, the spin-only magnetic moment value of the inner-orbital complex that absorbs light at shortest wavelength is ____B.M. [nearest integer]
Solution: