Q.
A thermodynamic system is taken from an original state $D$ to an intermediate state $E$ by the linear process shown in the figure. Its volume is then reduced to the original volume from $E$ to $F$ by an isobaric process. The total work done by the gas from $D$ to $E$ to $F$ will be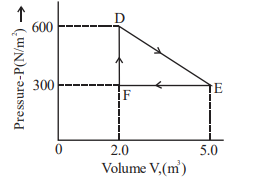
Solution: