Q.
A student measures pressure of a gas in a container using a mercury manometer and she also measures atmospheric pressure using a mercury barometer. She gave following representation
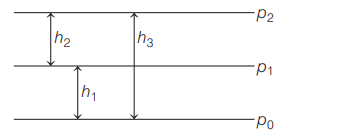
If $p_{1}=$ atmospheric pressure,
and $p_{2}=$ absolute pressure, then
Mechanical Properties of Fluids
Solution: