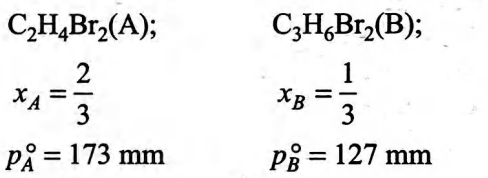Q.
A solution is prepared containing a $2: 1$ mol ratio of dibromoethane $\left( C _{2} H _{4} Br _{2}\right)$ and dibromopropane $\left( C _{3} H _{6} Br _{2}\right)$. What is total vapor pressure of the solution assuming ideal behaviour?
Solutions
Solution: